When the need is aortic, the solution is Artivion.
Our intentional focus on the aorta and collaboration with the world’s foremost cardiac and vascular surgeons allow us to leverage our combined expertise in the development of new, innovative, life-changing aortic-centric technologies. Explore our end-to-end aortic-centric product portfolio.
*Note: All products and indications are not available/approved in all markets. Please contact your local Artivion representative for details.
Select Your Market
Heart Valve Solutions
On-X®
Aortic Heart Valve
On-X®
Mitral Heart Valve
On-X®
Ascending Aortic Prosthesis
Chord-X®
Mitral Chordal Replacement Products
CryoValve® SG
Pulmonary Human Heart Valve
CryoValve®
Aortic Allograft
Allografts
Cardiac & Vascular
CryoValve® SG
Pulmonary Human Heart Valve
CryoValve®
Aortic Allograft
CryoPatch® SG
Pulmonary Patch
CryoGraft®
Descending Thoracic Aorta
CryoArtery®
Aortoiliac Artery
CryoArtery®
Femoral Artery
CryoVein®
Saphenous Vein
CryoVein®
Femoral Vein
CryoVein® PC
Pediatric Conduit
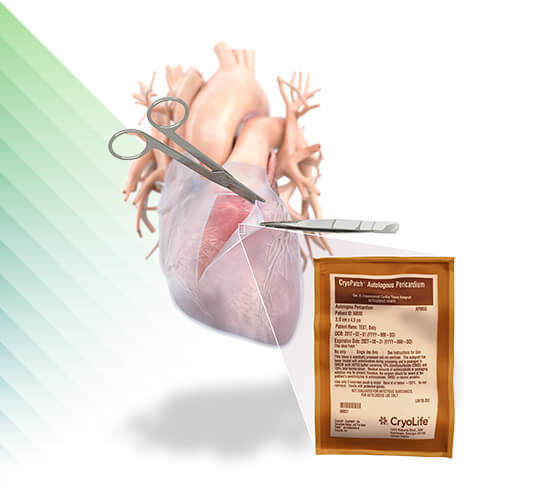
CryoPatch®
Autologous Pericardium
Surgical Sealant
BioGlue®
Surgical Adhesive
Cardiac and Vascular Ancillary Solutions
PhotoFix®
Decellularized Bovine Pericardium
CarbonAid® & CarbonMini®
CO2 Diffuser
Heart Valve Solutions
Aortic Arch Solutions
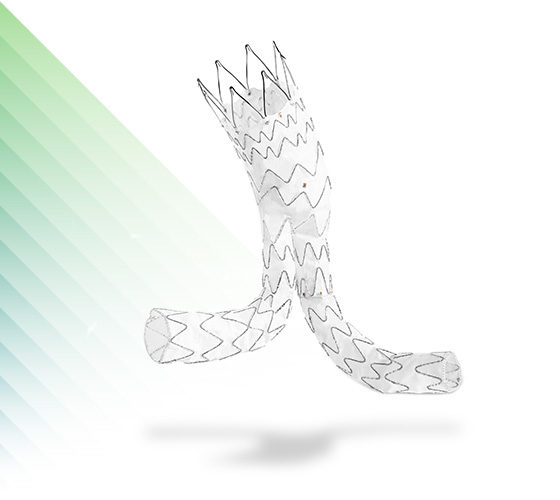
AMDS™
Hybrid Prosthesis
E-vita® Open Neo
Hybrid Stent Graft System
NEXUS®
Aortic Arch Stent Graft System
NEXUS DUO™
Aortic Arch Stent Graft System
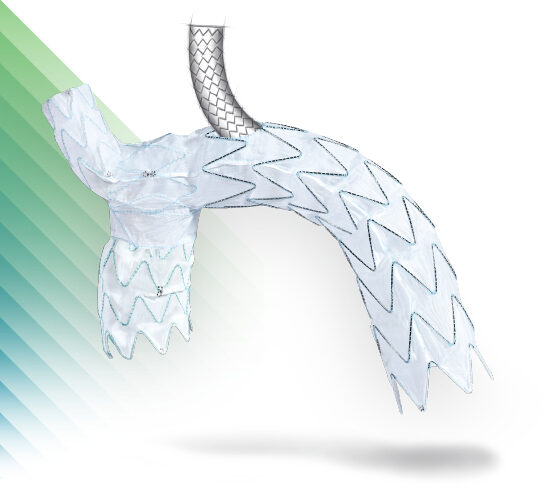
Thoracoabdominal Solutions
Artivex™
Thoracic Extension Stent Graft System
E-nside™ TAAA
Multibranch Stent Graft System
Abdominal Aorta Stent Grafts
Peripheral Aorta Stent Grafts
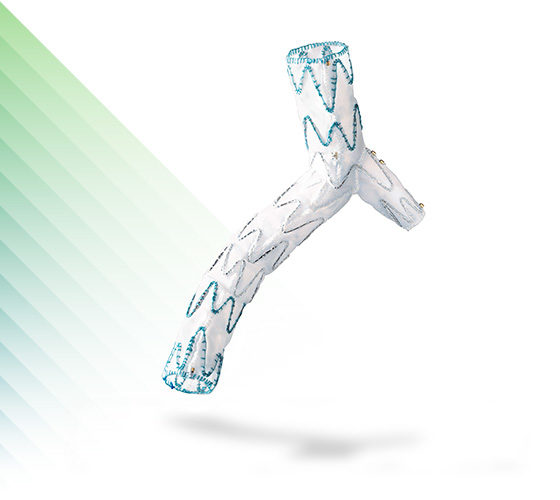
E-liac™
Stent Graft System
Surgical Sealant
BioGlue®
Surgical Adhesive
Cardiac and Vascular Ancillary Solutions
E-xtra Design Engineering
With our E-xtra Design MultiBranch Stent Graft System, we offer the option of having individual configurations that are tailor-made for the patient’s anatomy in the thoracoabdominal aorta. We have dedicated teams to help with planning, sizing, and implantation, so we can deliver your custom solution within 3 weeks of your request.*
*NOTE: This service is not available in the U.S. Delivery times vary by geography.
Over 5,000 successful implantations since the start of the program in 2012