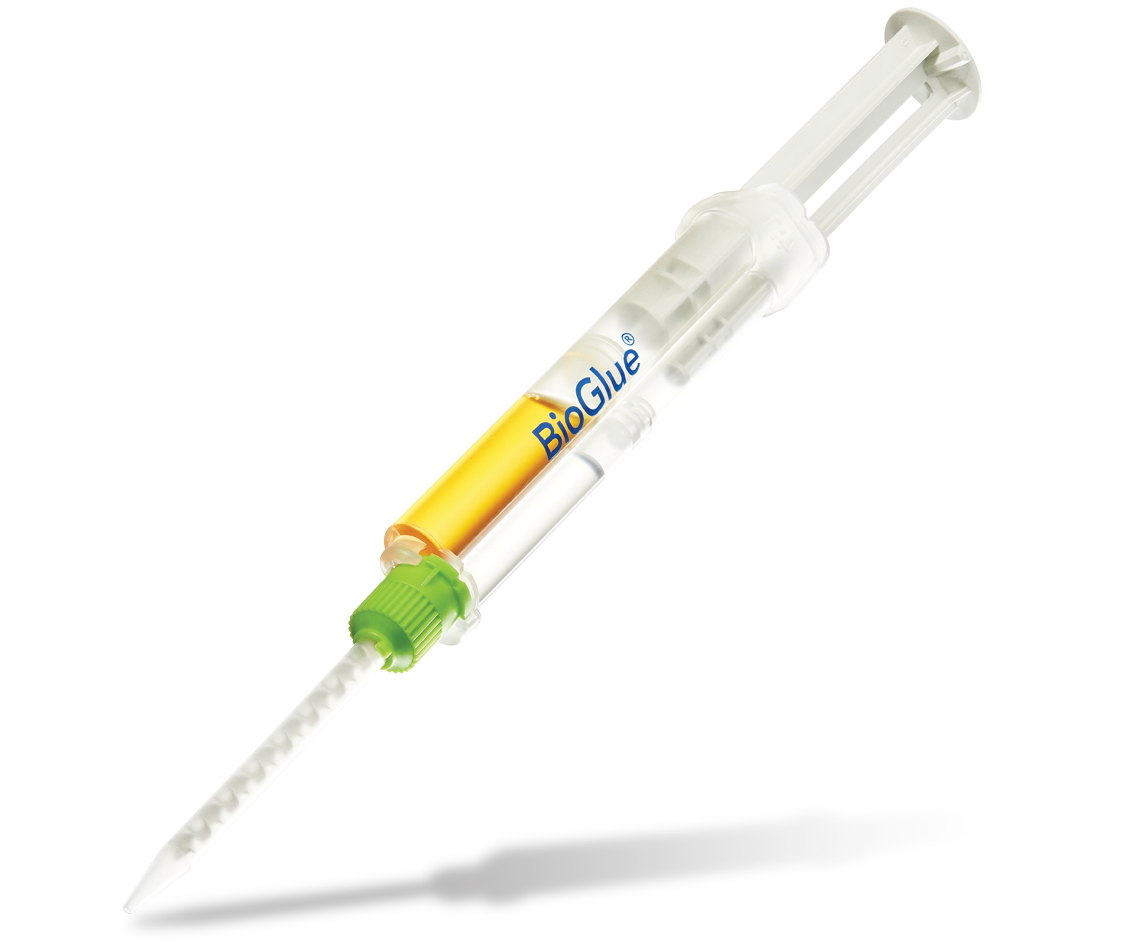
BioGlue®
SURGICAL ADHESIVE
Reinforcing Tissue,
Reinforcing Results.
Product Highlights
- The only surgical sealant approved to seal, adhere and reinforce tissue.1
- The strongest surgical sealant on the market.2
- There are over 500 published clinical and preclinical papers about BioGlue.3
Product Overview
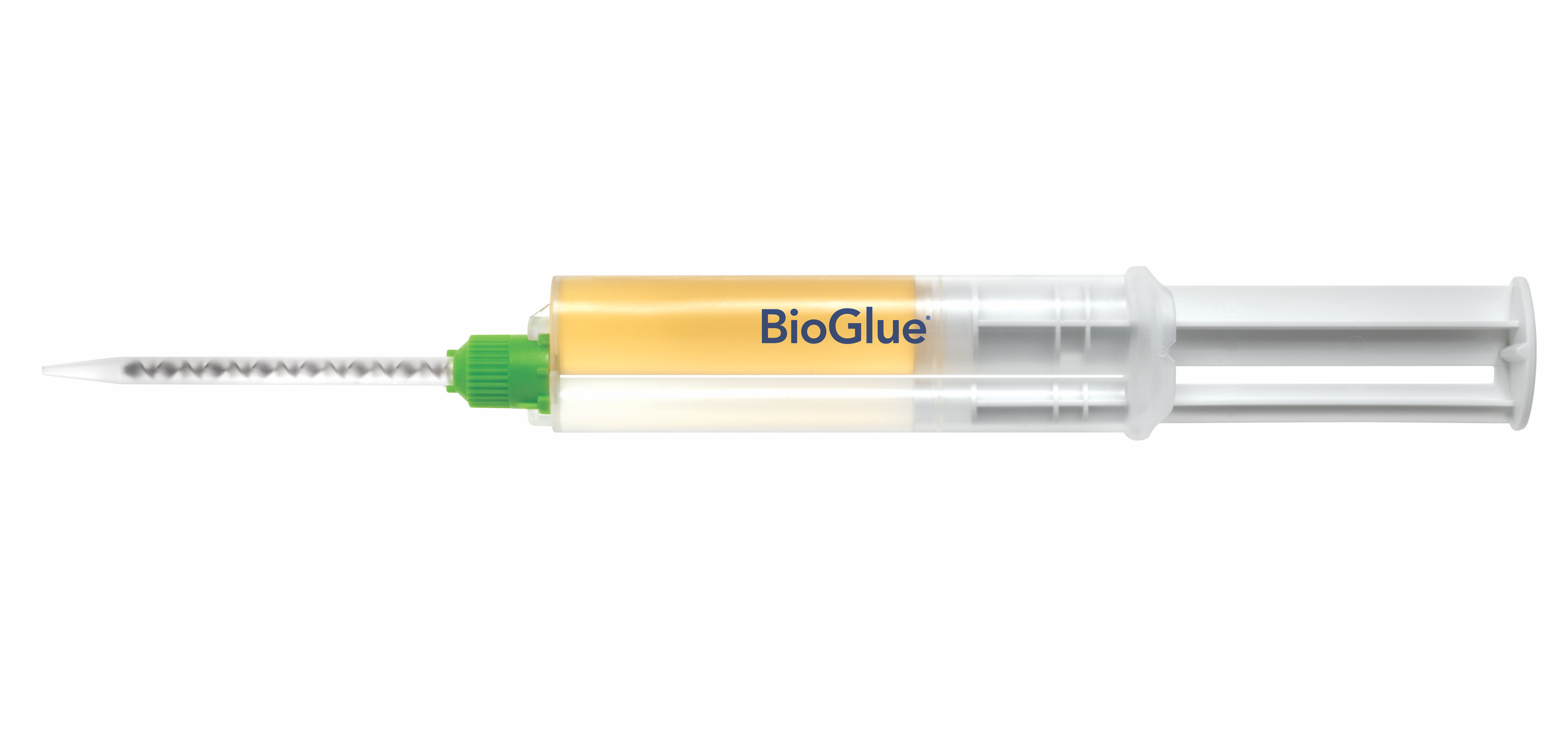
1. Pre-filled BioGlue Syringe
Specifically engineered to utilize a two chamber syringe which provide optimal ratio of its two components.
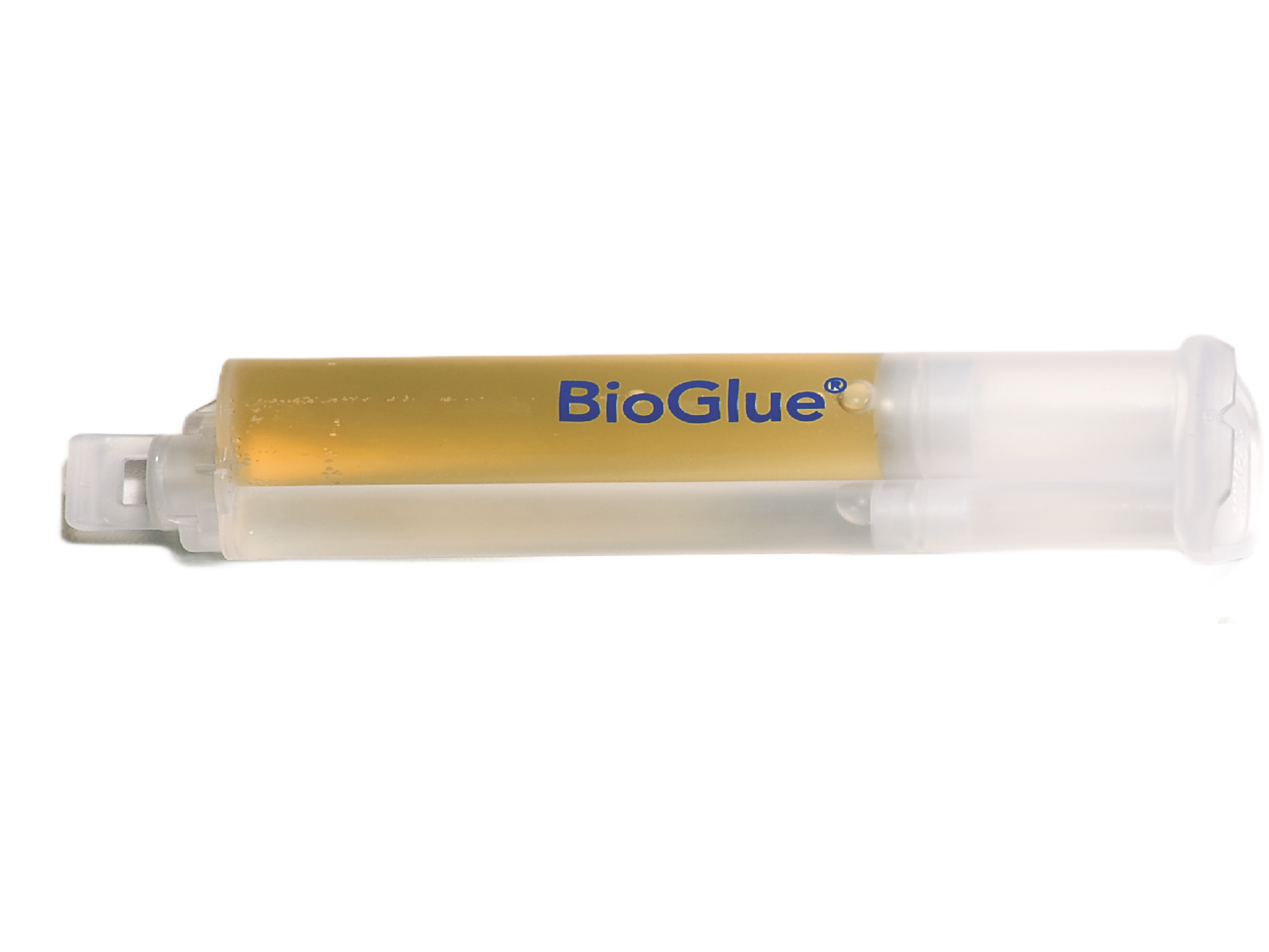
2. Syringe Plunger
Enables BioGlue to be ready to use in seconds.
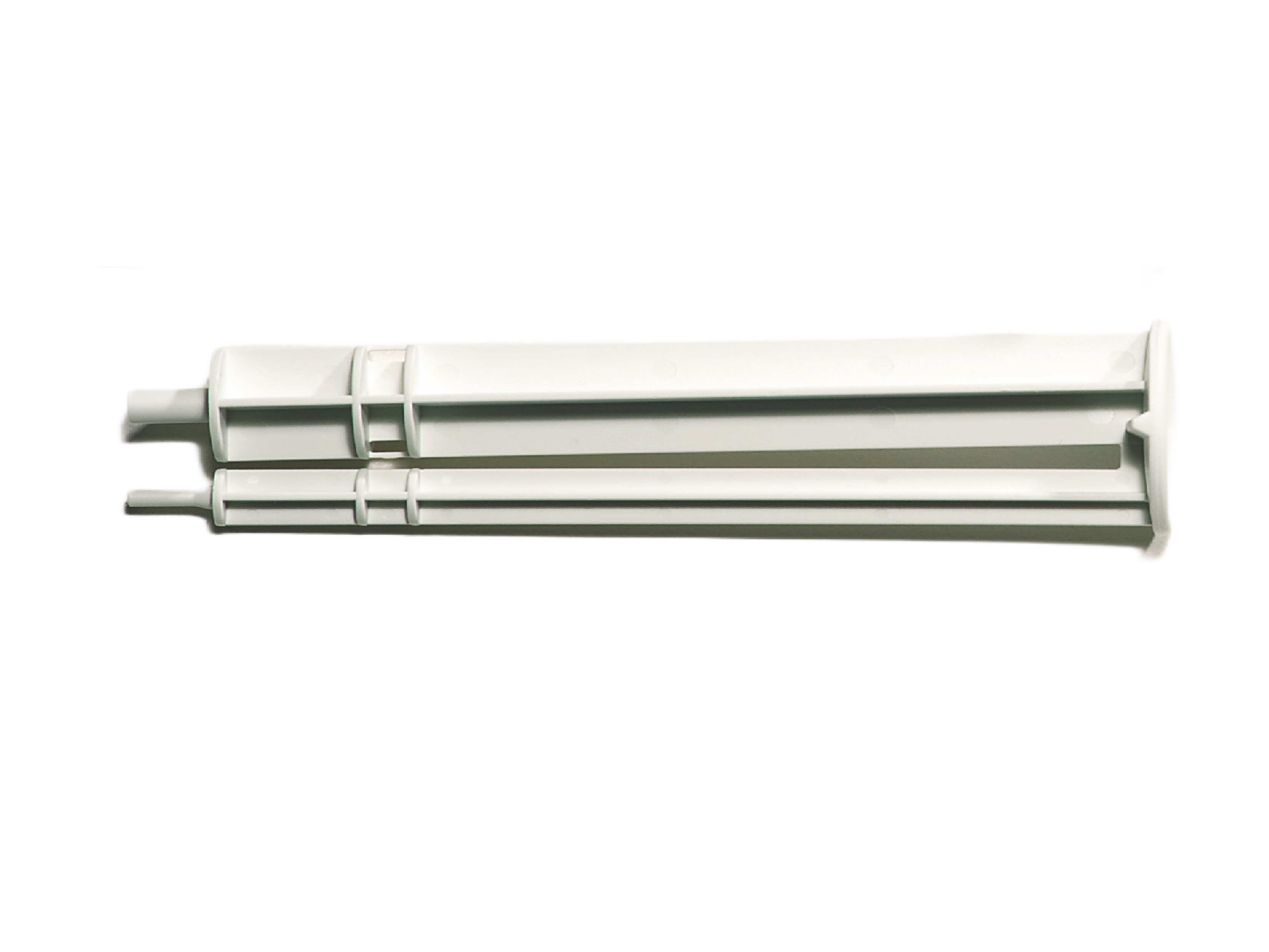
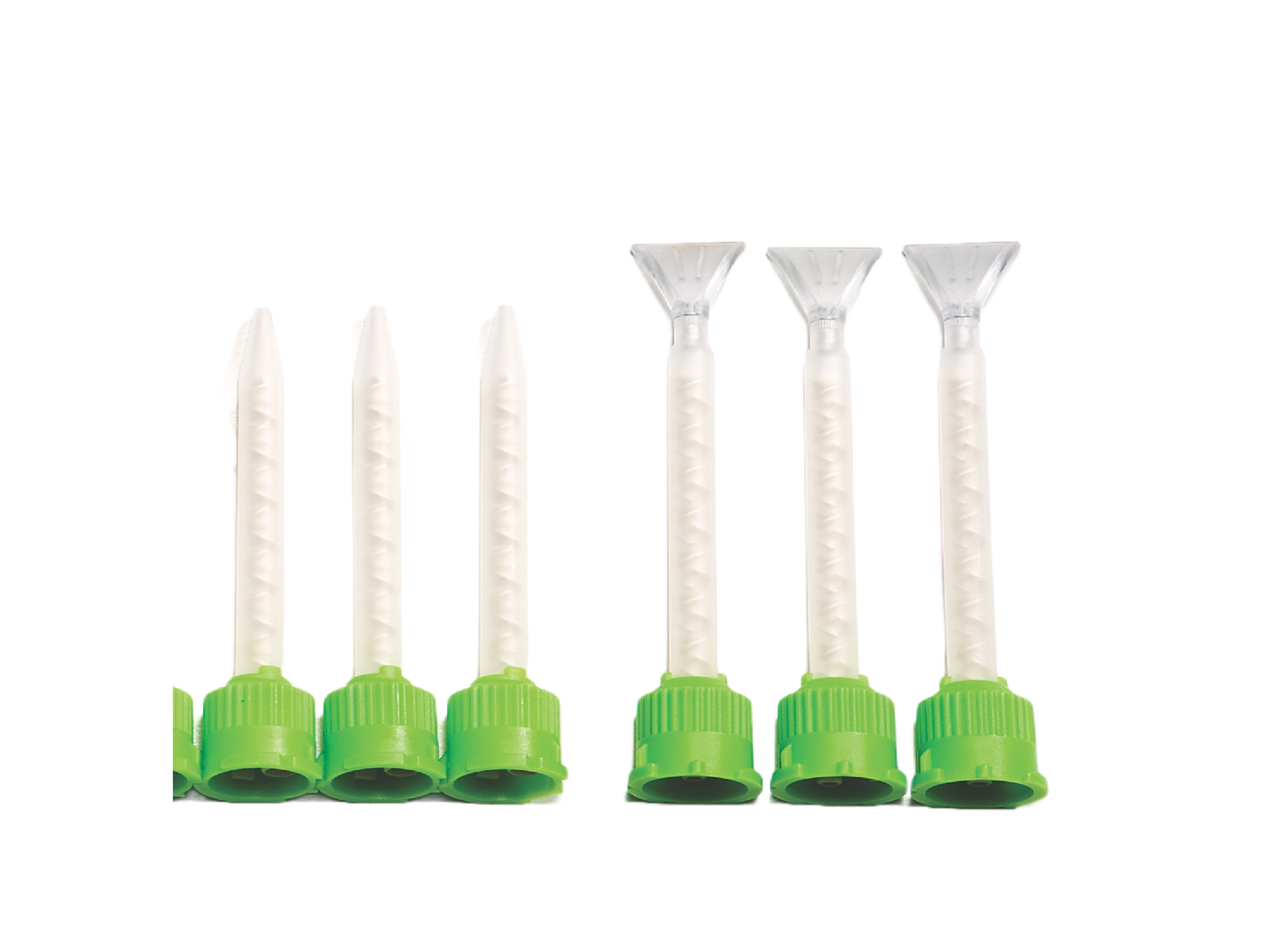
3. Syringe Applicator Tips & Spreader Tip
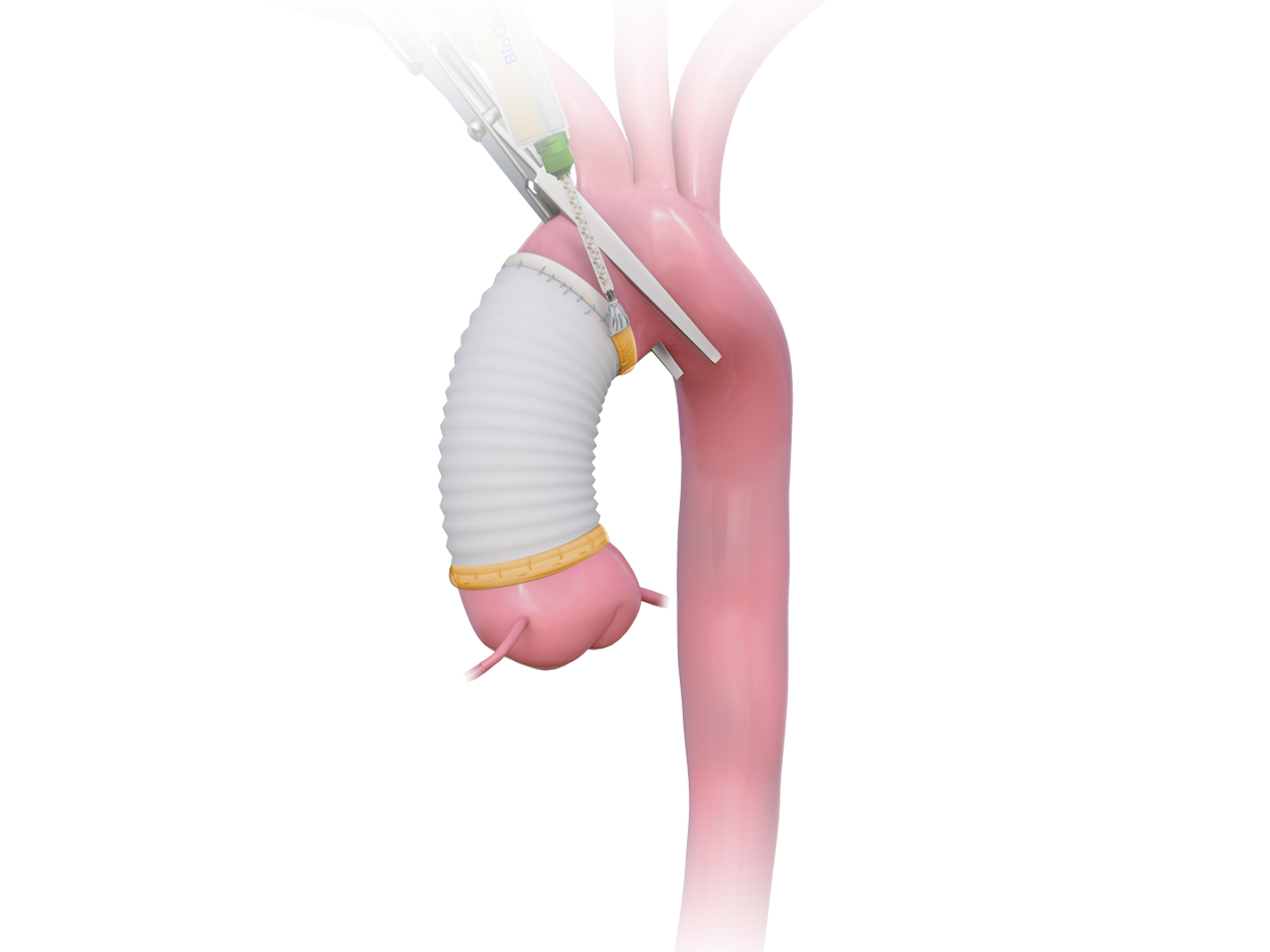
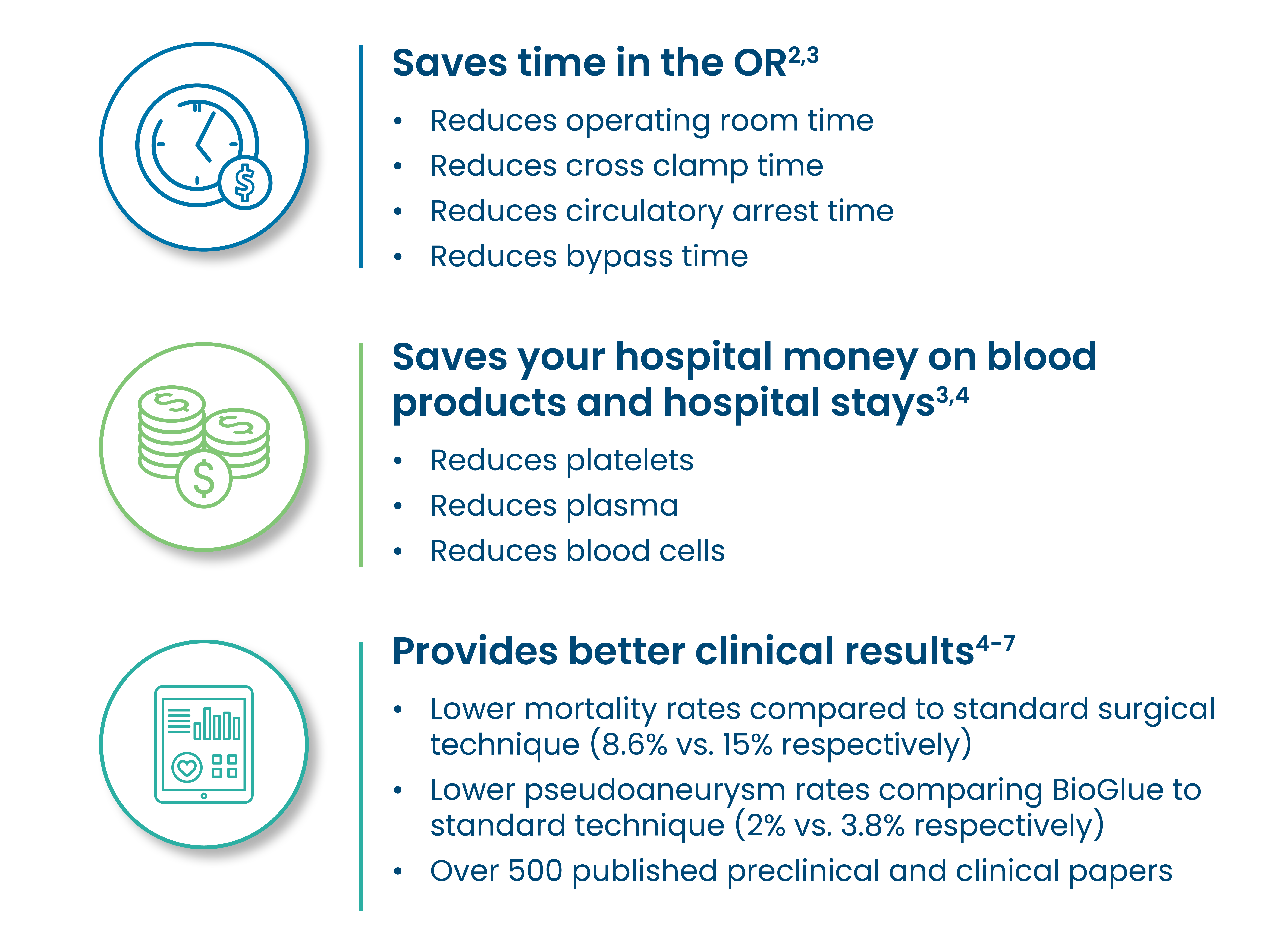
The use of BioGlue helped to facilitate a minimal reliance on blood products and a low mortality rate.1
The use of BioGlue reinforces friable tissue, facilitating anastomotic repair when tissues are fragile because of age or disease state.2
Clinical Evidence
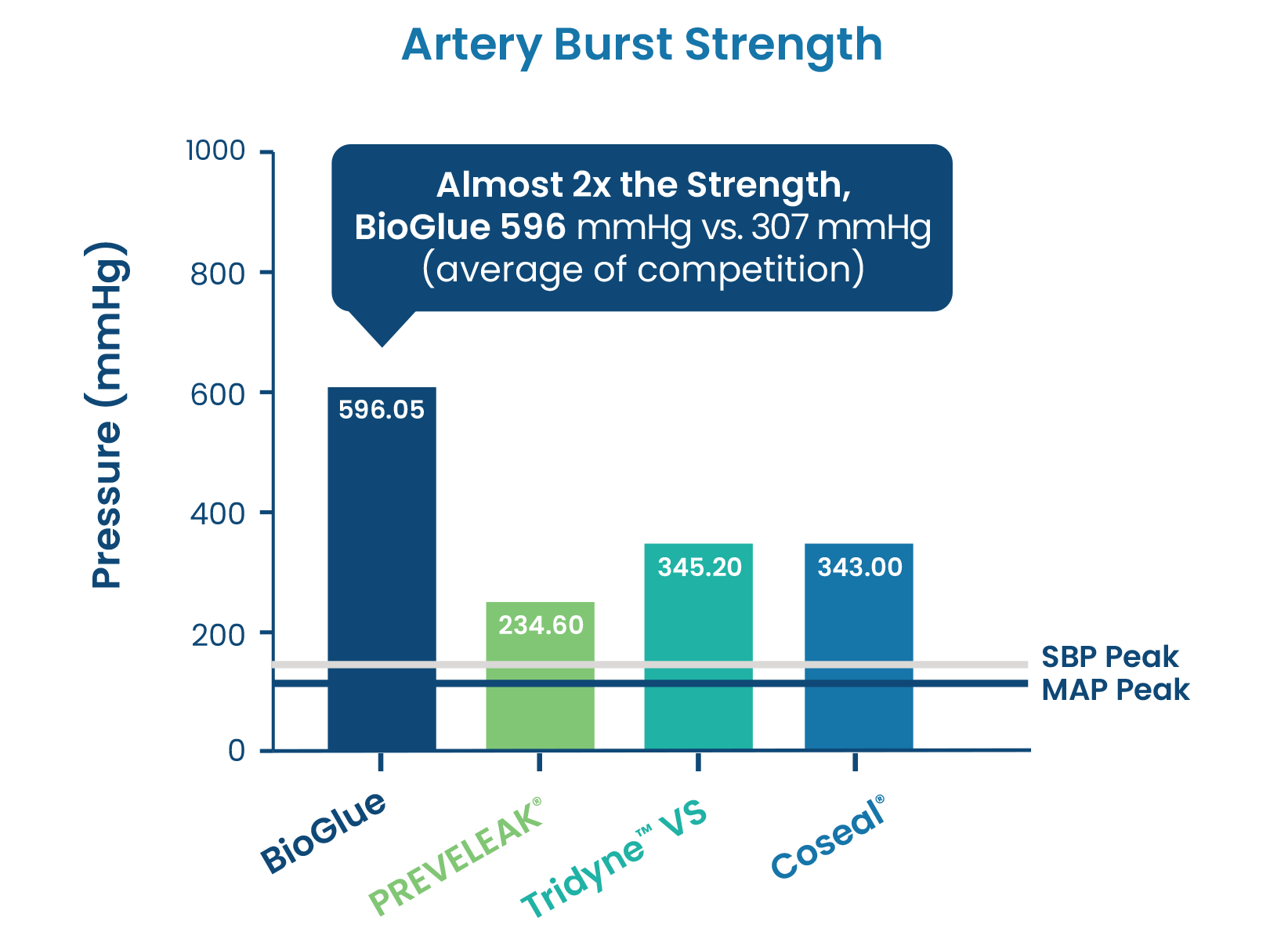
Artery Burst Tests
Artery Burst Tests Show BioGlue® is Significantly Stronger Than PREVELEAK®, Coseal®, and Tridyne™ VS.1
Reinforcing why BioGlue...
- Is the ideal sealant
- Is the only surgical sealant approved to seal, adhere and reinforce tissue8
- Is the gold standard in cardiac & vascular surgery
Additional Resources
Explore additional resources for BioGlue below. For further information or to contact a sales associate in your area, contact us.
Product Highlights
- BioGlue Summary of Safety and Effectiveness.
- Murdock M H, et. al. (2019). Cytocompatibility and mechanical properties of surgical sealants for cardiovascular applications. J Thorac Cardiovasc Surg, 157(1), 176-183.
- Artivion Internal BioGlue Bibliography.
Physician Quotes
- Fehrenbacher J W, et. al. (2006). Use of BioGlue in aortic surgery: Proper application techniques and results in 92 patients. Heart Surg Forum, 9(5), E794-799.
- Coselli J S, et. al. (2003). Prospective randomized study of a protein-based tissue adhesive used as a hemostatic and structural adjunct in cardiac and vascular anastomotic repair procedures. J Am Coll Surg, 197(2), 243-252.
Clinical Evidence
- Murdock M H, et. al. (2019). Cytocompatibility and mechanical properties of surgical sealants for cardiovascular applications. J Thorac Cardiovasc Surg, 157(1), 176-183.
- Feier, H. et. al. (2019). The influence of albumin/glutaraldehyde sealant in early results after acute type A aortic dissection. REV.CHIM.(Bucharest), 70(6).
- Chao, H. et. al. (2003). BioGlue: albumin/glutaraldehyde sealant in cardiac surgery. J Card Surg, 18(6), 500-503.
- Fehrenbacher J W, et. al. (2006). Use of BioGlue in aortic surgery: Proper application techniques and results in 92 patients. Heart Surg Forum, 9(5), E794-799.
- Internal BioGlue Bibliography
- Weiner, J. et. al. (2011). Role of bovine serum albumin-glutaraldehyde glue in the formation of anastomatic pseudoaneurysms. J Card Surg, 26(1), 76-81.
- Bavaria J E, et. al. (2002). Advances in the Treatment of Acute Type A Dissection: An Integrated Approach. Ann Thorac Surg, 74, S1848-1852.
- Bioglue Summary of Safety and Effectiveness
Product Video
- Fehrenbacher J W, et. al. (2006). Use of BioGlue in aortic surgery: Proper application techniques and results in 92 patients. Heart Surg Forum, 9(5), E794-799.
- Bavaria J E, et. al. (2002). Advances in the Treatment of Acute Type A Dissection: An Integrated Approach. Ann Thorac Surg, 74, S1848-1852.
- Artivion Internal BioGlue Bibliography
All products and indications are not available/approved in all markets. All trademarks are owned by Artivion, Inc. or its subsidiaries. On-X Life Technologies, Inc., Jotec GmbH, and Ascyrus Medical GmbH are wholly owned subsidiaries of Artivion, Inc. MLENG1610.000. (2023-04)
| Artivion, Inc., 1655 Roberts Blvd NW, Kennesaw, GA 30144, US |

